When a thermodynamic process occurs the change of state will happen. the process will Change from initial state to final state. the change in state in the process occurs in two ways. Here, the thermodynamic properties will undergo some changes to change the state or to complete the process. there are two ways of the thermodynamic process and these are the reversible or irreversible process.
Reversible process or Reversibility
A reversible process is a thermodynamic process which is capable of attaining its initial state by following the same path by which the process reaches to final state of the process from its initial state to complete the process.
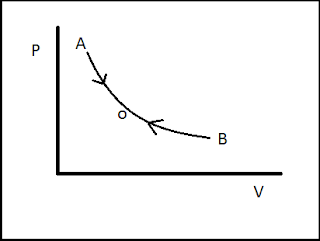
As shown in the graph the initial state of the system is A. when the thermodynamic process changes its state from A to B to complete the process the system will follow the path A-O-B. If the process is reversible then it will have the capability to get its initial state by following the same path B-O-A. this is called reversibility of a thermodynamic process.
The reversible process is not a practical phenomenon because there is always some deviations in the process So it is a theoretical consideration.
Quasi-Static process is an example of reversible process, but one thing should be kept in mind that Quasi-static process is itself an assumption. To understand the reversible process and thermodynamics equilibrium quasi static process it is analyzed.
In the graph, an Irreversible process shown. In the system When the state is changed from state A to state B the process is completed. To complete this process the path which is taken is A-O-B. When the system will try to attain its initial state A, it has to follow a different path A-Q-B. this makes this process an Irreversible process and This is called irreversibility of a process.
There is a lot depends on the properties of the system because thermodynamic properties of the system decide reversibility and irreversibility of the thermodynamic process.
This is a short description of the reversible process and irreversible process. Thermodynamic analysis largely depends on these phenomena.
 |
| Reversible process |
The reversible process is not a practical phenomenon because there is always some deviations in the process So it is a theoretical consideration.
Quasi-Static process is an example of reversible process, but one thing should be kept in mind that Quasi-static process is itself an assumption. To understand the reversible process and thermodynamics equilibrium quasi static process it is analyzed.
Irreversible process or Irreversibility
Irreversible process is different from a reversible process. The system will be able to regain its initial state after a process but it has to take a different path than the process is completed previously.
 |
| Irreversible process |
There is a lot depends on the properties of the system because thermodynamic properties of the system decide reversibility and irreversibility of the thermodynamic process.
This is a short description of the reversible process and irreversible process. Thermodynamic analysis largely depends on these phenomena.

cool
ReplyDeletehey dear,
ReplyDeletei m so happy because your post are wonderful and really useful for me,thanks dear for sharing…
https://www.lukhidiamond.com/LOOSE-DIAMONDS